We saw my oncologist yesterday. We see him every three weeks. My chemo-immunotherapy cycles are 3 weeks long – weeks 1 and 2 I receive treatment, week 3 is a break from treatment, but we see the oncologist after I get bloodwork done. So far these appointments have been primarily dedicated to my reporting any significant side effects (none really, except some fatigue and ringing in my ears) and him reviewing my bloodwork to determine whether I can proceed to the next cycle.
I’m about to begin cycle 6 of 8 total (assuming my platelet count improves over the weekend). In other words, we’re getting near the end. So, last time we met with him, we started pressing him on what comes next. The standard protocol would be to continue only with the durvalumab (immunotherapy) once a month and to drop the chemo (gemcitabine and cisplatin). Some patients (a small percentage) have gone years on just immunotherapy without seeing disease progression (i.e. the tumor growing back or the cancer spreading) or have even had their tumors shrink. But some have only gone months and then the tumor starts to grow again. So, I am not sure about just following the standard protocol. I have mixed feelings. On the one hand it would be a relief to go only once/month for treatment (with scans every 3 months, I think, to check on tumor size). It would feel like getting my life back a little. On the other hand, my goal is to shrink the tumor as much as possible so surgical removal becomes feasible; “stabilization” (the tumor doesn’t grow) doesn’t feel like enough to me. In any case, my husband I and were just happy to have started that conversation with my oncologist — it felt like we opened the door to future, more exhaustive conversations about potential treatment paths.
But back to yesterday. More information had just come in about the molecular analysis of my tumor. And it turns out the tumor may have an additional, very rare mutation called an NRG1 fusion. Less than 1% of cholangiocarcinoma patients have this mutation. I say my tumor “may have” this mutation because apparently identifying mutations is a trickier, more nuanced process than I realized. I guess it’s not always a matter of yes or no — the genomic analysis can require some interpretation. Also, it seems that this particular mutation cannot be identified through a DNA analysis of the tumor, only an RNA analysis, which not all cancer hospitals do (or have the ability to do), but mine can and does. And they use a special technique involving a laser to cut away the useless material in a biopsy (biopsy material doesn’t just contain samples of the tumor, it also contains fat, dead cells, and other junk that can get in the way of genomic analysis) to ensure the best possible tumor tissue for molecular sequencing. In addition, at this hospital they have two groups that do the molecular analysis – the “science” group and the “clinical” group. And the science group has determined that my tumor does have this mutation, but they are waiting on the clinical report to see if they came to the same conclusion. So, it’s a little up in the air right now.
But my oncologist said he couldn’t help himself in sharing this news (perhaps prematurely) because if the tumor does have this mutation, it is “highly targetable,” meaning there is an effective drug for it, zenocutuzumab. He was very excited, and showed us on his computer screen a 2025 article in the New England Journal of Medicine about the efficacy of zenocutuzumab (below is a screen shot of information about NRG1 fusion).
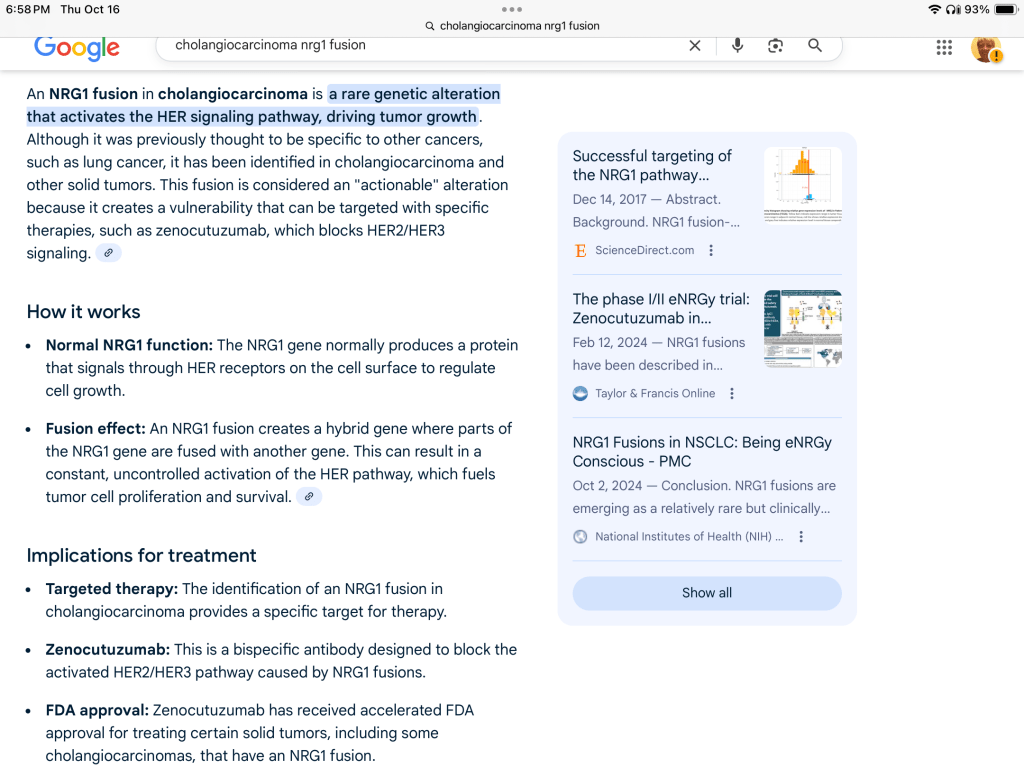
And it gets more “exciting” than that — a real cliffhanger. The FDA has approved this drug (https://www.onclive.com/view/fda-grants-fast-track-designation-to-zenocutuzumab-in-nrg1-cholangiocarcinoma?fbclid=IwdGRleANosk1leHRuA2FlbQIxMQABHo5yn6moK3xFjKRwDtPTPpOsbW2v_3F6afEobXSlnmVmrxqDJm3ZyP8XKwM6_aem_u3zo2X8EfkdOcp3UhmkUBQ ), but Health Canada has not, so it is not currently available here. Getting access to it would require (1) requesting that the company that makes it provide it to my hospital, and (2) requesting that Health Canada approve my hospital giving it to me under a “compassionate use” provision (or something like that).
So, a lot of “ifs” at this point — if my tumor has the NRG1 fusion, if the pharmaceutical company will give the drug to us, if Health Canada will approve its use. But even with all these ifs my oncologist seemed very excited. I think he was excited not only about the possibility of having another tool in the treatment toolkit, but also about having a case involving an extremely rare mutation that he will present to the hospital “Tumor Board” — the group of oncologists, researchers, and surgeons who meet regularly to discuss cancer cases. I have suddenly become a much more interesting patient.
Leave a comment